Download pdf: Soil texture and pH effects on potash and phosphorus availability (1.10M)
pdf 1.10M
Soil texture and pH effects on potash and phosphorus availability
January 2015
Dr Paul Hargreaves, SRUC, Crichton, Dumfries.
The application, use, efficiency and loss of nutrients including K and P can vary with soil type. Soil type is generally determined by the texture of the soil, which is a measure of the proportions of the following three particles: sand, classified as having a particle size between 0.05mm and 2.0mm, silt 0.05mm to 0.002 mm and clay <0.002mm. The soil texture triangle shown in Figure 1 is an indication of the proportions of sand, silt and clay found in different soil types as determined by laboratory analysis.
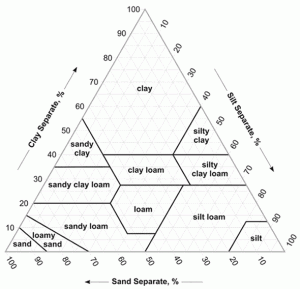
As the amounts of the three particles vary, the texture and potential ability of the soil to support various crops will change. A light sandy soil containing a greater proportion of sand particles will be more freely draining, as the larger sand particles leave gaps that allow the water to flow through more easily than a heavier clay soil with finer particles and reduced space for water movement.
Potassium (K) salts dissolve easily and the K ions in solution diffuse through the soil from areas of high K concentrations to areas of low K concentrations. The rhizosphere, the area of soil around the surface of crop roots, usually has low K concentrations due to the uptake of K ions from the soil solution into the roots. The moisture content of the soil is important for the successful movement of K for use by the crop; the wetter the soil the more mobile the K ions will be.
- Heavy soils are those that contain more clay and these tend to have large reserves of K, which are available to the crop and produce higher soil K indices on analysis. However, some of these large reserves of K can be held by the lattice structure of the clay minerals and are only slowly released over time.
Some clay soils contain high levels of potash and their clay minerals can release K into available forms for crop uptake each year. e.g. 50kg K2O/ha. Hence they need less K applied. Examples of these potash releasing clays are: Chalky boulder clay, Gault clay, Weald clay, Kimmeridge clay, Oxford clay, Lias clay, Oolitic clay – these are calcareous clays.
Clay soils which do not release much potash in this way: Carboniferous clays (the others). - Sandy soils contain low levels of clay and analysis of these soils reveals low levels of plant extractable K. This is because it is not easily held within the sandy soil. As a consequence of the larger sand particles water moves more easily through these soils and the potential for the loss of K from soil (i.e. leaching) is greater. More consideration needs to be given to the timing and amounts of K applied for the crop on these soils.
As the majority of the plant extractable K, in these lighter sandy soils, will be provided by fertiliser additions, a strategy of little and often for K applications may be best. Usually some is applied in the seed bed plus additional spring applications with the N top dressing. Remember some applications can be as organic manures.
P and K availability in the soil
Potassium (K) is similar to Phosphorus (P), as it is found in different availability amounts or “pools” within the soil. The status of the availability changes as it moves between pools.
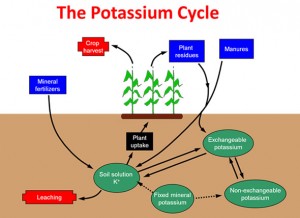 The pools for K are described as: soil solution (very available), exchangeable (less available), non-exchangeable (hardly available) and fixed (rarely available) (Figure 2). The dissolved K ions in soil solution are readily taken up by crop roots and generally comprise between 2 to 5 mg/l in normal agricultural soils.
The pools for K are described as: soil solution (very available), exchangeable (less available), non-exchangeable (hardly available) and fixed (rarely available) (Figure 2). The dissolved K ions in soil solution are readily taken up by crop roots and generally comprise between 2 to 5 mg/l in normal agricultural soils.
For P the available forms are generally present as orthophosphate but only very small amounts can be held within the soil water, typically only about 0.05 mg/l or 1 – 2 % of total P. Therefore the release and mobilisation of insoluble and fixed forms of P is even more important for increasing soil P availability to the crop.
As the clay particles/lattices usually have a negative surface charge, they hold onto positively charged particles, such as K+ ions. These K+ ions, held on the surface of clay particles, can be removed or exchanged relatively easily by other similar charged ions, and hence are available to move into the soil solution to be taken up by the crop roots quite easily.
The non-exchangeable pool contains less available K+ ions that have become strongly attached to the clay particles as they are trapped in between the lattice structure of the clays and are only available when these layers are opened up, with for example, increased soil moisture or with changing soil pH. These ions are only released slowly from the edges and exposed areas of the lattices, usually by weathering through the season ie. drying and wetting or freezing and thawing.
The fixed or mineral pool contains K held within the mineral crystalline matrix and so is only very slowly released for crop use by the weathering of these materials. This process is most significant in the potash releasing clays, which have been referred to earlier.
As a further consideration, P added to the soil as a fertiliser will considerably increase the concentration of P in the soil solution and the readily available pool and is therefore available for crop uptake. However, higher P applications, and therefore concentrations, can encourage more P to precipitate out of solution through reactions with charged molecules and soil particles. This P then becomes part of the fixed pool and is much less available. The more time the P is retained without moving back into the soluble pool the greater the P will become fixed in the soil and unavailable for immediate crop use. Therefore continued application of P to soils that are already high in P could be encouraging more P to be locked up within the soil, which is wasteful and may contribute to environmental damage when soil erosion occurs.
K and High Magnesium Soils
 Potassium availability may be affected by soils with very high Magnesium (Mg) levels. Where the concentration (mg/l) of soil Mg is more than twice that of soil K, potash availability to the crop may be less than expected from the soil analysis. This is due to an antagonistic interaction at the crop root for up take.
Potassium availability may be affected by soils with very high Magnesium (Mg) levels. Where the concentration (mg/l) of soil Mg is more than twice that of soil K, potash availability to the crop may be less than expected from the soil analysis. This is due to an antagonistic interaction at the crop root for up take.
There are more Mg ions competing for the sites on the root and so less of the K ions are taken up. If this occurs, higher applications of K may be required to overcome the imbalance and satisfy the plants’ needs. The application of additional Ca (as gypsum) to reduce the soil Mg is an alternative approach, but it must be spread with care, as a big excess of Ca could unintentionally reduce some of the K (it would be lost through leaching) as well as the Mg.
Influence of Soil pH
The soil pH can affect the availability of both K and P in the soil.
In alkaline soils (soil pH greater than 7) Ca is the dominant positive ion for reactions for both K and P. The greater the concentration of Ca ions in the soil, the greater the increase in K availability, as the Ca will displace the K from the clay lattice and make it more available in solution for the plant (Table 1). Differently, the formation of Ca and P compounds decreases their solubility and therefore reduces the availability of P.
In acidic soils the reduction in Ca will reduce the amount of K displaced into solution and hence reduce the availability to crop roots. In the case of P, especially with soil pH less than 5.5, Al is the more common ion to react, as well as Fe and some Ca. These gradually result in very insoluble P containing compounds that are generally not available to plants. Hence, maintaining soil pH between 6 and 7 will generally result in the most efficient use of K and P (Table 1).
| Table 1. Decrease in K and P availability as soil acidity increases. | |||
| pH Values | K | P | |
| pH 6.0 | Increasing acidity ↓ |
100% | 52% |
| pH 5.5 | 77% | 48% | |
| pH 5.0 | 52% | 34% | |
Summary
Understanding the texture of the soil you are working with and the effect this can have on the retention and availability of both K and P is important. This, along with the soil moisture and pH of the soil, can have a considerable effect on the efficiency of the K and P applied.

